


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-10-2020
Date: 26-8-2019
Date: 15-5-2017
|
Ethers are widely used as solvents for a variety of organic compounds and reactions, suggesting that they are relatively unreactive themselves. Indeed, with the exception of the alkanes, cycloalkanes and fluorocarbons, ethers are probably the least reactive, common class of organic compounds. The inert nature of the ethers relative to the alcohols is undoubtedly due to the absence of the reactive O–H bond.
The most common reaction of ethers is cleavage of the C–O bond by strong acids. This may occur by SN1 or E1 mechanisms for 3º-alkyl groups or by an SN2 mechanism for 1º-alkyl groups. Some examples are shown in the following diagram. The conjugate acid of the ether is an intermediate in all these reactions, just as conjugate acids were intermediates in certain alcohol reactions.
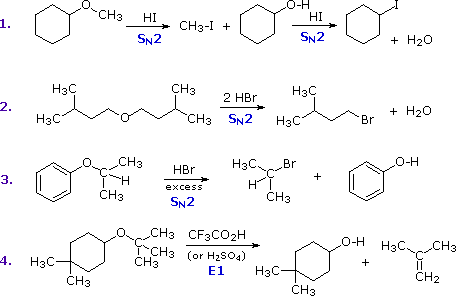
The first two reactions proceed by a sequence of SN2 steps in which the iodide or bromide anion displaces an alcohol in the first step, and then converts the conjugate acid of that alcohol to an alkyl halide in the second. Since SN2 reactions are favored at least hindered sites, the methyl group in example #1 is cleaved first. The 2º-alkyl group in example #3 is probably cleaved by an SN2 mechanism, but the SN1 alternative cannot be ruled out. The phenol formed in this reaction does not react further, since SN2, SN1 and E1 reactions do not take place on aromatic rings. The last example shows the cleavage of a 3º-alkyl group by a strong acid. Acids having poorly nucleophilic conjugate bases are often chosen for this purpose so that E1 products are favored. The reaction shown here (#4) is the reverse of the tert-butyl ether preparation described earlier.
Ethers in which oxygen is bonded to 1º- and 2º-alkyl groups are subject to peroxide formation in the presence of air (gaseous oxygen). This reaction presents an additional hazard to the use of these flammable solvents, since peroxides decompose explosively when heated or struck. The mechanism of peroxide formation is believed to be free radical in nature (note that molecular oxygen has two unpaired electrons).
| R–O–CH(CH3)2 + O2 |  |
R–O–C(CH3)2–O–O–H a peroxide |



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|