


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-10-2020
Date: 14-9-2018
Date: 17-9-2018
|
One of the largest and most diverse classes of reactions is composed of nucleophilic additions to a carbonyl group. Both reversible and irreversible addition reactions have been described, and in all cases the initial step involved covalent bonding of a nucleophile to the electrophilic carbon atom of the carbonyl group. As noted earlier, conjugation of a double bond to a carbonyl group transmits the electrophilic character of the carbonyl carbon to the beta-carbon of the double bond. A resonance description of this transmission is shown below. From this formula it should be clear that nucleophiles may bond either at the carbonyl carbon, as for any aldehyde, ketone or carboxylic acid derivative, or at the beta-carbon. These two modes of reaction are referred to as 1,2-addition and 1,4-addition respectively.
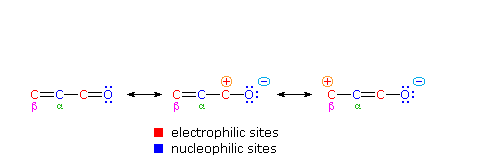
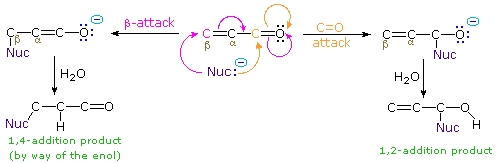
The nucleophile in this scheme is shown with a negative charge, which is neutralized in the addition products by treatment with water. Neutral nucleophiles such as 1º and 2º-amines may also add in the same manner, and do not require a neutralization step. The term "1,4-addition" is applied to the product of conjugate addition (initial nucleophile bonding at the beta-carbon) because the product initially formed is presumably the unstable enol tautomer.
Reversible addition reactions of nitrogen, oxygen and sulfur nucleophiles to unsaturated carbonyl and nitrile compounds normally give 1,4-addition products rather than their 1,2-addition isomers. This preference for conjugate addition may be attributed in part to the thermodynamic advantage of addition reactions to carbon-carbon double bonds over additions to a carbonyl function. This factor was noted earlier in the chapter on aldehydes and ketones. Although nucleophilic addition reactions to alkenes are usually slow, conjugation with a carbonyl or nitrile function vinylagously activates the beta-carbon, resulting in rapid addition. It is likely that rapid 1,2-addition occurs as well, but because it is reversible, the thermodynamically favored 1,4-product accumulates. Several examples of these conjugative addition reactions are given below.
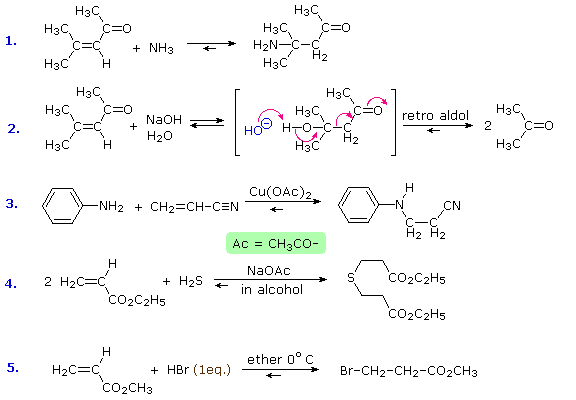
The reaction of 4-methyl-3-penten-2-one with hydroxide ion (# 2) is interesting because the 1,4-addition product is the aldol product from acetone. A retro (or reverse) aldol reaction generates acetone as the chief product. The third and fourth reactions demonstrate the use of acetate salts as catalysts for some conjugative additions, and the last reaction is an acid-catalyzed 1,4-addition (bromide anion is the nucleophile).
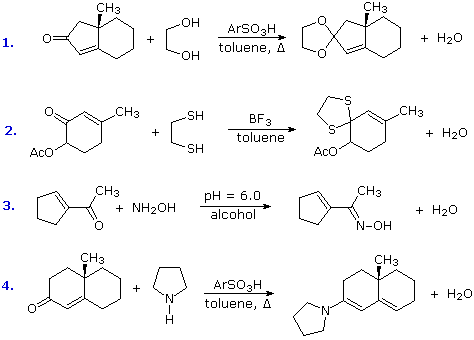
Some typical aldehyde and ketone substitution reactions that proceed from 1,2-addition intermediates still take place in the expected manner when conjugated double bonds are present. Most of these involve a final dehydration that is only possible if an initial 1,2-addition has occurred. As demonstrated by the following examples, acetals (# 1 & 2), imine derivatives (# 3) and enamines (# 4) can all be prepared in the usual way.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|