


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-10-2020
Date: 3-8-2019
Date: 28-8-2018
|
An important body of chemical reactions, differing from ionic or free radical reactions in a number of respects, has been recognized and extensively studied. Among the characteristics shared by these reactions, three in particular set them apart.:
1. They are relatively unaffected by solvent changes, the presence of radical initiators or scavenging reagents, or (with some exceptions) by electrophilic or nucleophilic catalysts.
2. They proceed by a simultaneous (concerted) series of bond breaking and bond making events in a single kinetic step, often with high stereospecificity.
3. In agreement with 1 & 2, no ionic, free radical or other discernible intermediates lie on the reaction path.
Since reactions of this kind often proceed by nearly simultaneous reorganization of bonding electron pairs by way of cyclic transition states, they have been termed pericyclic reactions. The four principle classes of pericyclic reactions are termed: Cycloaddition, Electrocyclic, Sigmatropic, and Ene Reactions. A general illustration of each class will be displayed by clicking on the following diagram. The cycloaddition and ene reactions are shown in their intermolecular format. Corresponding intramolecular reactions, which create an additional ring, are well known.
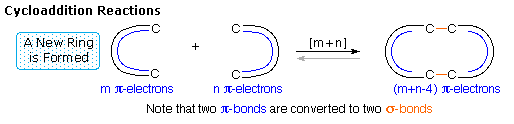



All these reactions are potentially reversible (note the gray arrows). The reverse of a cycloaddition is called cycloreversion and proceeds by a ring cleavage and conversion of two sigma-bonds to two pi-bonds. The electrocyclic reaction shown above is a ring forming process. The reverse electerocyclic ring opening reaction proceeds by converting a sigma-bond to a pi-bond. As shown, the retro ene reaction cleaves an unsaturated compound into two unsaturated fragments. Finally, sigmatropic bond shifts may involve a simple migrating group, as shown in the example above, or may take place between two pi-electron systems (e.g. the Cope rearrangement).
The general descriptions shown above provide a basis for reaction classification, but care must be taken to assure that a given transformation is truly concerted. Unfortunately, this is not a trivial determination, often requiring a combination of isotope labeling and stereochemical studies to arrive at a plausible conclusion. There is also a subtle distinction to be made between a synchronous reaction in which all bond-making and bond-breaking events take place in unison, and a multi-stage concerted process in which some events precede others without generating an intermediate state.
Although some pericyclic reactions occur spontaneously, most require the introduction of energy in the form of heat or light, with a remarkable product dependence on the source of energy used.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|