


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 4-10-2020
Date: 28-8-2018
Date: 21-1-2022
|
A radical is an atomic or molecular species having an unpaired, or odd, electron. Some radicals, such as nitric oxide (NO), are relatively stable, but most are so reactive that their isolation and long-term study is not possible under normal laboratory conditions. The electrons in most stable organic compounds are paired in atomic or molecular orbitals, so the total electron count is an even number. Molecular oxygen (O2) is a rare example of a stable biradical (two unpaired electrons having the same spin), with an even number of electrons.
Early chemists used the term "radical" for nomenclature purposes, much as we now use the term "group". Many doubted that such open-valenced species could exist, although there was circumstantial evidence for their participation in gas phase reactions. Credit for the first isolation and characterization of a "free radical" goes to Moses Gomberg, a young instructor at the University of Michigan. In 1900 Gomberg attempted a synthesis of hexaphenylethane by reacting triphenylmethyl chloride with finely divided metals such as silver and zinc. When air was excluded from the reaction, he obtained a yellow solution, the color of which darkened reversibly on heating and cooling. This solution yielded a colorless, crystalline C38H30 hydrocarbon which Gomberg assumed to be hexaphenylethane.


If the yellow solution was exposed to air (or oxygen) a C38H30O2 peroxide was obtained, and identified by reduction to the known alcohol, triphenylmethanol. In a similar fashion the yellow solution reacted with iodine to produce triphenylmethyl iodide. Gomberg concluded that the colored solutions contained reactive triphenylmethyl free radicals, formed by thermal dissociation of their dimer (Keq = 2 • 10–4 at 25º C). The exceptional stability of this carbon radical is attributed to odd electron delocalization into the three phenyl rings. Discrete Kekule formulas demonstrate that this benzyl-like delocalization places the electron on ortho and para carbons, but not on meta carbons. Clicking on the diagram a third time will display this delocalization in a general way.
The resonance structures drawn here may give the impression that the triphenylmethyl radical is planar (flat). Actually the phenyl groups are turned by about 35º, producing a shape similar to a three bladed propellor. Despite this twist, the p-pi orbital overlap is still over 80%, so the electron delocalization is not seriously diminished. To see a model of this unusual radical .
More than fifty years later, the reactive dimer of triphenylmethyl radical was shown to be the para-coupled compound drawn above and not hexaphenylethane. The steric crowding of phenyl groups in the simple ethane dimer is apparently so severe that bonding between two 3º-carbon atoms is prohibited. Since the electron delocalization noted above places radical character at the para carbons of the phenyl groups, bonding to this relatively unhindered location is preferred, although at the cost of one benzene ring's aromaticity. If the para-locations are themselves hindered by large meta substituents, then an unstable hexaarylethane may actually be formed.
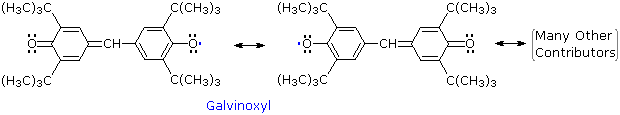
Other relatively stable radicals, such as galvinoxyl have been prepared and studied. These species usually owe their stability to a combination of odd electron delocalization and steric hindrance to dimerization, as the ortho tert-butyl groups in galvinoxyl demonstrate. The term "free radical" is now loosely applied to all radical intermediates, stabilized or not.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
الزائرون يحيون ليلة الجمعة الأخيرة من شهر ربيع الآخر عند مرقد أبي الفضل العبّاس (عليه السلام)
|
|
|